Overview: In reporting numerical results, it is important to include the correct number of significant digits. While determining the correct number of digits to include is a straightforward process, beginning students often overlook this important detail. Here we outline the rules involved in determining the appropriate number of digits to include when reporting results of calculations and experimental measurements.
- Bromine Test Results
- Results In Chemistry Publication Fees
- Results In Chemistry
- Impact Factor Of Chemistry Journals
- Results In Chemistry
Chemical symbols are abbreviations used in chemistry for chemical elements, functional groups and chemical compounds. Element symbols for chemical elements normally consist of one or two letters from the Latin alphabet and are written with the first letter capitalised. Results that are high or low might have the letter (H) or (L) after the number, or may be printed to the side or in a different column to call attention to the abnormal result. Again, getting a copy of your lab results lets you compare your numbers to the normal ranges and makes it easier to ask questions about the results and what they mean.
Skills:
- Reporting scientific results with the appropriate number of significant digits.
New terms:
- Significant Figures
- Precision
- Accuracy
Defining the Terms Used to Discuss Significant Figures
Significant Figures: The number of digits used to express a measured or calculated quantity.
By using significant figures, we can show how precise a number is. If we express a number beyond the place to which we have actually measured (and are therefore certain of), we compromise the integrity of what this number is representing. It is important after learning and understanding significant figures to use them properly throughout your scientific career.

Accuracy: Refers to how closely individual measurements agree with the correct or true value.
Digits that are Significant
- Non-zero digits are always significant.
- Any zeros between two non-zero digits are significant.
- A final zero or trailing zeros in the decimal portion ONLY are significant.
Examples:
How many significant figures are in:1. 12.548, 2. 0.00335, 3. 504.70, 4. 4000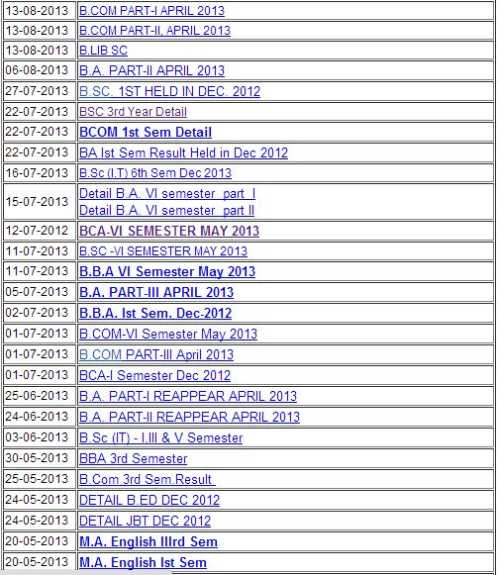
- There are 5. All numbers are significant.
- There are 3. The zeros are simply placeholders and locate the decimal. They are not trailing zeros. They are not significant.
- There are 5. The two zeros are not simply placeholders. One is between two significant digits and the other is a final, trailing zero in the decimal portion. Hence, they are both significant.
- This is a bit confusing. It is somewhere between 1 and 4. In order to clarify, we need to convert this to scientific notation. If it were 4 x 103, there is one significant figure. If it were 4.000 x 103, then there are 4 significant figures.
Rules for Using Significant Figures
- For addition and subtraction, the answer should have the same number of decimal places as the term with the fewest decimal places.
- For multiplication and division, the answer should have the same number of significant figures as the term with the fewest number of significant figures.
- In multi-step calculations, you may round at each step or only at the end.
- Exact numbers, such as integers, are treated as if they have an infinite number of significant figures.
- In calculations, round up if the first digit to be discarded is greater than 5 and round down if it is below 5. If the first discarded digit is 5, then round up if a nonzero digit follows it, round down if it is followed by a zero.
More Examples:
Addition and Subtraction.12.793 + 4.58 + 3.25794 = 20.63094- With significant figures it is 20.63 since 4.58 has 2 decimal places, which is the least number of decimal places.
Multiplication and Division.56.937/0.46 = 130.29782609
- With significant figures, the final value should be reported as 1.3 x 102 since 0.46 has only 2 significant figures. Notice that 130 would be ambiguous, so scientific notation is necessary in this situation.
Tidiness at the end of a calculation.
So you have carried out a calculation that requires a series of seven or eight mathematical operations and at the end, after punching everything into your calculator, you see the result '14.87569810512..'. Kulot style boy. The question you should ask yourself is how many digits to include when reporting your final answer.
It is at this point that you must refer back to the quality of the data you were given (i.e., how many significant digits are included with the given data). We illustrate this here with one final example.
Three scientists determine the mass of
Bromine Test Results
in the sample, how will each do this in a way that reflects the precision of the instrumentation they are using? The three scientists all use the atomic masses suggested by IUPAC (International Union of Pure and Applied Chemistry), which are included in the table below.Scientist B | |||
given data |
|
|
|
|
|
| |
Why? | The balance used for the mass determination limits the result to 3 significant digits. | The quality of the instrumentation is better, than that used by Scientist A, but the result is still limited to only 4 significant digits. | Why not 6 significant digits in the reported result? This time the answer is limited by the uncertainty in the atomic mass of Fe, which is known to 5 significant digits! |
This brings up an interesting question. Why is the atomic mass of chlorine known to 6 significant figures, while that of iron is only known to 5 significant figures? Click here for an explanation.
Results In Chemistry Publication Fees
More Examples
- Examples of rounding to the correct number of significant figures with a 5 as the first non-significant figure
- Round 4.7475 to 4 significant figures: 4.7475 becomes 4.748 because the first non-significant digit is 5, and we round the last significant figure up to 6 to make it even.
- Round 4.7465 to 4 significant figures: 4.7465 is 4.746 because the first non-significant digit is 5 and since the last significant digit is even, we leave it alone.
- An example of a calculation where you can 'lose' significant figures doing an operation.
The mass of 19F is 18.99840 u. How much mass is converted to energy when a 19F atom is assembled from its constituent protons, neutrons, and electrons?
19F 9 p+ + 9 e- + 10 n0
Results In Chemistry
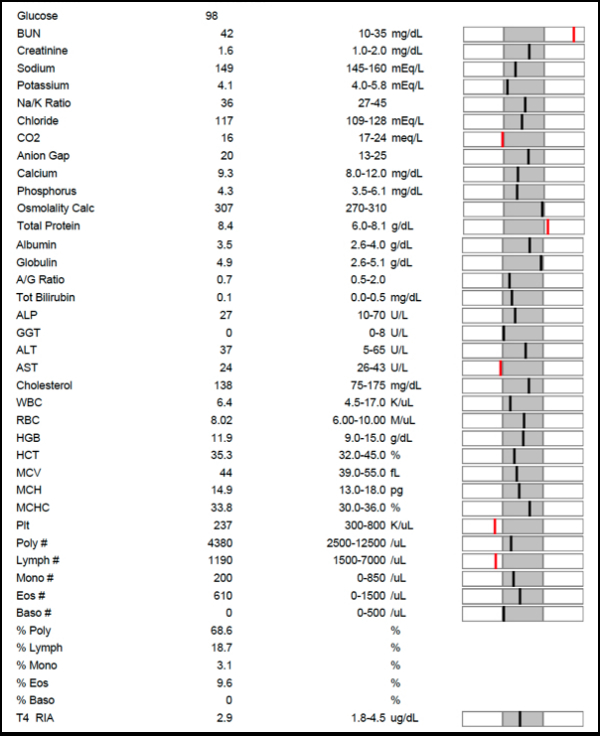
Accuracy: Refers to how closely individual measurements agree with the correct or true value.
Digits that are Significant
- Non-zero digits are always significant.
- Any zeros between two non-zero digits are significant.
- A final zero or trailing zeros in the decimal portion ONLY are significant.
Examples:
How many significant figures are in:1. 12.548, 2. 0.00335, 3. 504.70, 4. 4000- There are 5. All numbers are significant.
- There are 3. The zeros are simply placeholders and locate the decimal. They are not trailing zeros. They are not significant.
- There are 5. The two zeros are not simply placeholders. One is between two significant digits and the other is a final, trailing zero in the decimal portion. Hence, they are both significant.
- This is a bit confusing. It is somewhere between 1 and 4. In order to clarify, we need to convert this to scientific notation. If it were 4 x 103, there is one significant figure. If it were 4.000 x 103, then there are 4 significant figures.
Rules for Using Significant Figures
- For addition and subtraction, the answer should have the same number of decimal places as the term with the fewest decimal places.
- For multiplication and division, the answer should have the same number of significant figures as the term with the fewest number of significant figures.
- In multi-step calculations, you may round at each step or only at the end.
- Exact numbers, such as integers, are treated as if they have an infinite number of significant figures.
- In calculations, round up if the first digit to be discarded is greater than 5 and round down if it is below 5. If the first discarded digit is 5, then round up if a nonzero digit follows it, round down if it is followed by a zero.
More Examples:
Addition and Subtraction.12.793 + 4.58 + 3.25794 = 20.63094- With significant figures it is 20.63 since 4.58 has 2 decimal places, which is the least number of decimal places.
Multiplication and Division.56.937/0.46 = 130.29782609
- With significant figures, the final value should be reported as 1.3 x 102 since 0.46 has only 2 significant figures. Notice that 130 would be ambiguous, so scientific notation is necessary in this situation.
Tidiness at the end of a calculation.
So you have carried out a calculation that requires a series of seven or eight mathematical operations and at the end, after punching everything into your calculator, you see the result '14.87569810512..'. Kulot style boy. The question you should ask yourself is how many digits to include when reporting your final answer.
It is at this point that you must refer back to the quality of the data you were given (i.e., how many significant digits are included with the given data). We illustrate this here with one final example.
Three scientists determine the mass of the samesample of FeCl3. Scientist A works in a field laboratory and carries a portable balance for determining the sample mass, the balance can determine masses to the nearest +/- 0.1 g. Scientist B has a better, but still somewhat crude balance, which reports the mass to the nearest +/- 0.01 g. Scientist C has a balance, like the analytical balances you will find in chemistry laboratories at WU, that can determine sample masses to the nearest +/- 0.0001 g. If each scientist wants to indicate the total number of moles of FeCl3Bromine Test Results
in the sample, how will each do this in a way that reflects the precision of the instrumentation they are using? The three scientists all use the atomic masses suggested by IUPAC (International Union of Pure and Applied Chemistry), which are included in the table below.Scientist B | |||
given data |
|
|
|
|
|
| |
Why? | The balance used for the mass determination limits the result to 3 significant digits. | The quality of the instrumentation is better, than that used by Scientist A, but the result is still limited to only 4 significant digits. | Why not 6 significant digits in the reported result? This time the answer is limited by the uncertainty in the atomic mass of Fe, which is known to 5 significant digits! |
This brings up an interesting question. Why is the atomic mass of chlorine known to 6 significant figures, while that of iron is only known to 5 significant figures? Click here for an explanation.
Results In Chemistry Publication Fees
More Examples
- Examples of rounding to the correct number of significant figures with a 5 as the first non-significant figure
- Round 4.7475 to 4 significant figures: 4.7475 becomes 4.748 because the first non-significant digit is 5, and we round the last significant figure up to 6 to make it even.
- Round 4.7465 to 4 significant figures: 4.7465 is 4.746 because the first non-significant digit is 5 and since the last significant digit is even, we leave it alone.
- An example of a calculation where you can 'lose' significant figures doing an operation.
The mass of 19F is 18.99840 u. How much mass is converted to energy when a 19F atom is assembled from its constituent protons, neutrons, and electrons?
19F 9 p+ + 9 e- + 10 n0
Results In Chemistry
Impact Factor Of Chemistry Journals
Return to the Chemistry Subject IndexBeilstein Test
The Beilstein test confirms the presence of a halogen in solution, although it does not distinguish between chlorine, bromine, or iodine. A copper wire is dipped into the halogen-containing solution and thrust into a flame. The copper oxide on the wire reacts with the organic halide to produce a copper-halide compound that gives a blue-green color to the flame.
Procedure: In the fume hood, clean a looped copper wire by thrusting it into the tip of the blue cone of a Bunsen burner flame until it glows (Figure 6.46a). Be sure to 'burn off' any residual liquid on the wire (make sure any green flames from previous tests are gone before you begin).
Allow the copper to cool to room temperature, then dip it into a test tube containing 5-10 drops of your sample, coating it as much as possible (Figure 6.46b). If the sample is a solid, adhere some of the solid to the copper wire by first wetting the wire with distilled water then touching it to the solid.
Results In Chemistry
Immediately plunge the wire with sample into the blue cone of the flame. A positive result is a green flame, although it might be short-lived and faint (it may be easier to see if the fume hood light is turned off). A negative result is the absence of this green color (Figure 6.46c+d).
